
ATF5 protein in untreated cells was set at 1.0. Relative ATF5 protein levels were determined as the ratio of 3×FLAG-ATF5 to β-galactosidase. 3×FLAG-ATF5 and β-galactosidase were analyzed by Western blot analysis using anti-FLAG and anti-β-galactosidase. After 48 h, cells were treated with IL-1β (0.1 ng/ml) for the indicated times. A, HepG2 cells were transiently cotransfected with 2 μg of pSV40–3×FLAG-ATF5 and 2 μg of pSV40-β-galactosidase as a control.

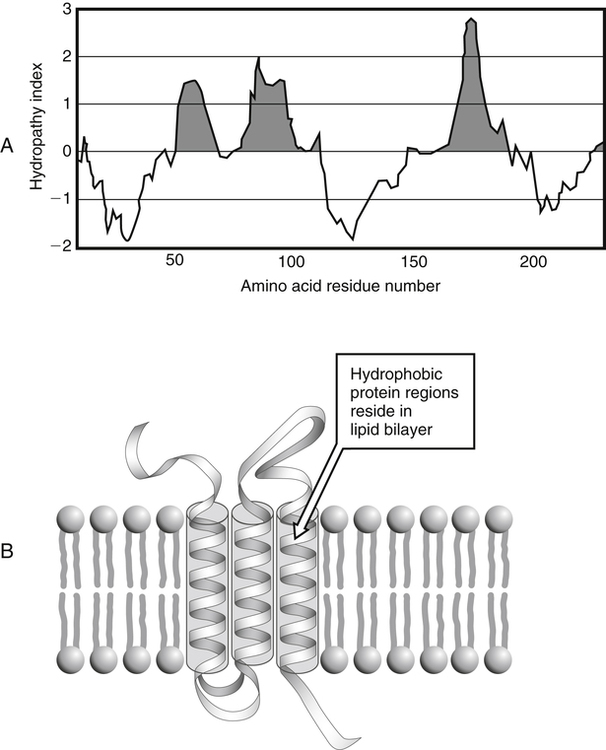

IL-1β increases ATF5 protein by enhancing stability. Heat Shock Protein Interleukin Protein Degradation Stress Response Transcription Factors. Our results show that the N-terminal hydrophobic amino acids play an important role in the regulation of ATF5 protein expression in IL-1β-mediated immune response and that ATF5 is a negative regulator for IL-1β-induced expression of SAA1 and SAA2 in HepG2 cells. ATF5 knockdown in HepG2 cells up-regulated the IL-1β-induced expression of the serum amyloid A 1 (SAA1) and SAA2 genes. Furthermore, IL-1β increased the translational efficiency of ATF5 mRNA via the 5' UTRα and phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α). The N-terminal domain rich in hydrophobic amino acids that is predicted to form a hydrophobic network was responsible for destabilization in steady-state conditions and served as an IL-1β response domain. In this study, we show that interleukin 1β (IL-1β), a proinflammatory cytokine, increases the expression of ATF5 protein in HepG2 hepatoma cells in part by stabilizing the ATF5 protein. The N-terminal amino acids contribute to the destabilization of the ATF5 protein in steady-state conditions and serve as a stabilization domain in the stress response after CdCl2 or NaAsO2 exposure. Shown at the right is the structure of serine.Activating transcription factor 5 (ATF5) is a stress-response transcription factor that responds to amino acid limitation and exposure to cadmium chloride (CdCl2) and sodium arsenite (NaAsO2). These amino acids are usually found at the surface of proteins, as discussed in the Proteins 2 module. These are serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr). Six amino acids have side chains that are polarīut not charged. This fact has important implications for proteins' tertiary structure (see the Proteins 2 module for a discussion of tertiary structure).

These side chains are composed mostly of carbon and hydrogen, have very small dipole moments, and tend to be repelled from water. Shown at the right is the structure of valine. Side chains are glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). The nine amino acids that have hydrophobic Amino acids are grouped according to what their side chains are like.


 0 kommentar(er)
0 kommentar(er)
